Gene Therapy
Gene therapy to regain life body function, reaching over to the cure of intractable diseases.

Jichi Medical University Regional Medical Center Oriental Medicine Department Specially appointed Professor
The function of the human body is becoming more understood at the gene level in the human cell. The mechanism for developing intractable disease for which the cause is not known is becoming clearer and detailed. Also the method to bring back the body function to normal at the gene level has been developed. This is gene therapy. It is a medical technology which aims to cure intractable diseases by introducing the genes from outside of the body and by regaining the lost bodily function. It has almost overcome intractable disease in the central nervous system that was previously difficult. Once gene therapy was considered not safe, however the innovation of medical technology has dispelled fears for the safety about gene therapy. Already the stage of research and development of gene therapy is shifting to commercial application and away from universities and laboratories We interviewed the Jichi Medical University Professor Shin-ichi Muramatsu who established numerous achievements in the study of gene therapy for intractable diseases in the central nervous system, in addition he also established a bio-tech company to accelerate its practical use, He was asked about the frontier of gene therapy and prospect of practical use.
– It is unique that you are researching the gene therapy in the department of oriental medicine. To the untrained eye you are in charge of both ends of medical technology.
It may seem strange indeed. But as a physician to treat neurological diseases, I believe that gene therapy and traditional Chinese medicine go together in harmony. Treatment for the diseases of the brain and the nerves had been unsuccessful with available methods. Because of that, the majority of treatment was relieving the symptoms such as numbness, dizziness and headache. Chinese medicine was effective for that. Actually I’m still using Chinese medicine for about 80% of our patients. I’ve been studying oriental medicine for over 30 years. Traditional Chinese medicine itself is one of the most important treatments and works well with other diseases. I’m a professor of the Department of Oriental medicine as I was most familiar with this on campus. I used to be the deputy chairman of Japan Society for Oriental Medicine.
However a novel medical technology has emerged that could cure neurological diseases in recent years. It was gene therapy. Not only relieving symptoms but also aiming to cure, I’m focusing on gene therapy research and development.
Supply for living features from outside
– I see, Oriental medicine was important as a symptomatic treatment and gene therapy was needed as a curative therapy. What neurological diseases do you intend to cure?
In Japan, we have a rapidly aging population, in 20 years 14.5% of the population will be aged 80 or older. As the population ages there will be more and more people with neurodegenerative diseases such as Parkinson’s, Alzheimer’s, amyotrophic lateral sclerosis (ALS) and multiple system atrophy (MSA). Neurodegenerative diseases are caused by certain nerve cells in the brain and spinal cord dying progressively. Symptoms of Parkinson’s disease are trembling hands stiff muscles, Alzheimer’s disease symptoms are decreased memory and judgement.
The World Health Organization (WHO) estimates that those who die because of neurodegenerative diseases will be the next following cancer and heart disease and that possibly neurodegenerative diseases will become the main cause of death. In an aging society the importance of neurodegenerative disease treatment will increase, therefore gene therapy will make a huge contribution to it.
– What is the gene therapy?
Genes contain information to make various proteins for the proper body motions, and for body maintenance and growth, these are required for life. In gene therapy by delivering genes into cells in the affected area from the outside of the body, the delivered genes recover the functions to produce the correct proteins.
There are various ways to deliver genes into cells and we adopt a method using a virus as a vector incorporated with the gene of interest. We make the viral vectors with the gene of interest to infect the target cells. Then, the virus transfects the gene of interest into host cells. The gene of interest which produces the required proteins can cure diseases.
Safety of gene therapy is significantly higher.
– Transfecting genes from the outside of the body by viral vectors sound anxious to me
You don’t have to worry too much.
Indeed, the history of gene therapy hasn’t been smooth. There are several types of vectors used for gene therapy such as retrovirus*1, adenovirus*2 or adeno-associated virus (AAV)*3. There was an accident with retrovirus which inserts the gene of interest in DNA. In that case, an off-target occurred and it activated the oncogenes. There was another case using adenovirus for the treatment of a specific monogenic disease. The patient died because of whole body inflammation due to a strong immune response. I think that many people had an impression from two accidents that gene therapy is a very risky treatment.
However that situation has changed since AAV vector technology has been in place. Many clinical studies of the gene therapy using AAV have been executed for hemophilia, retinal pigment degeneration, cystic fibrosis, etc. There are 173 cases in the world so far and there are no adverse effects caused by AAV vector. Of course we are also using AAV vector for our gene therapy research and development.
*1 Retrovirus: Organisms have two kinds of DNA and RNA genes, DNA; virus has only one of them. The virus that contains DNA is called DNA virus, and the virus containing RNA is called RNA virus. Retro virus is part of the RNA virus family it copies double stranded RNA as double stranded DNA then integrates into the host chromosomal DNA where it expresses itself. The size is 80~130nm. HIV and influenza are typical examples of retrovirus. The Moloney murein leukemia virus (MoMLV) is used as a vector. There are good features of the retroviral genes that maintain long term benefit to descendant cells, which enhance their long term expression.
*2 Adenovirus: Adenovirus is DNA virus with double stranded DNA. Size is 70~90nm. It is one of the viruses causing the common cold. Adenovirus vector infects many cell types and there are advantages in implementing large genes approximately 36 k base (retrovirus is 8 to 9 k base). K base is a unit showing how many ranges of one of four bases composing nucleic acid. For example 36 k base means 36000 bases consists of nucleic acid molecule. Sometimes you might see kb for k base. If the two polymers are paired like DNA, 1pair is considered to be 1 base. Sometimes it is indicated as 1base pair (bp) descendant cells, which enhance its long term expression.
*3 AAV: AAV is DNA virus with single stranded DNA which converts to double stranded DNA in the cell to express itself there. It’s not capable of self-growth, except when infected with other viruses such as adenovirus. For this reason it is highly safe. The size of this virus is 18~26nm. The size of the gene is about 5k base. Genes can be deployed in a terminal differentiation such as nerve cells; in addition, long term development is expected in non-dividing cells. On the other hand the size of the gene to be introduced is small for example 4.5 k base.
Challenge to Parkinson’s disease by genes producing enzymes in the body.
– You are saying that improvement of safety has boosted the practical use. Do you think you can get the result that you expect?
A significant effect has been confirmed in many clinical trials. I will show you some of the examples applied to neurological diseases, along with the principle treatments. First example is Parkinson’s disease.
Parkinson’s disease is caused by a lack of the neurotransmitter dopamine due to inability of dead nerve cells in the brain substantia nigra (a part of the brain nucleus). In healthy people, the substantia nigra neurons are stretched to the striatum (components of the brain involved in motor action), producing the dopamine, but in Parkinson’s disease, this is interrupted. It is a kind of engine oil to smoothly move the brain; you cannot supply dopamine from outside of the body by using medicines.
At first the theory of synthesized dopamine in the body is the following (Figure 1). In the end part of the nerve stretching out from substantia nigra, the amino acid tyrosine composes dopamine via a substance called L-dopa. Raw material tyrosine is supplied through the blood vessels from food consumption. In the process of synthesizing dopamine from tyrosine, two enzymes of TH (Tyrosine hydroxylase) and AADC (aromatic L amino acid decarboxylase) are necessary. By using TH, L-dopa is synthesized from tyrosine, and dopamine is synthesized from L-dopa by ADDC. Also, as a material to help TH function, tetrahydrobiopterin (BH4) is required, and to synthesize it in a short period of time, another enzyme called GCH (guanosine triphosphate cyclohydrolase I) is necessary. In other words, to make dopamine from raw materials, three enzymes (TH.ADDC.GCH) are needed.
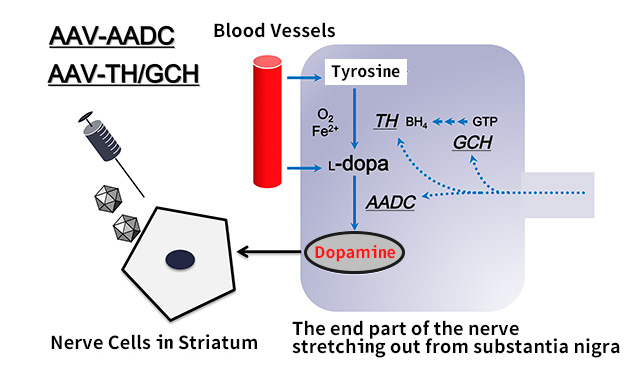
However when Parkinson’s disease has advanced, dopamine produced nerve terminals no longer exist, in addition the activity of the enzymes for synthesis of dopamine also falls. In our gene therapy we integrate the required genes for synthesis of dopamine into striatal neurons, using AAV vector.
However we thought it would be too difficult to add all functions at once to produce the inherent enzymes. That’s why we started clinical trials with the targeted treatment only adding the ability to synthesize dopamine from L-dopa. You can’t supply dopamine from outside of the body but L-dopa is possible using medicine. In fact in the therapy for Parkinson’s disease, L-dopa is administrated in drug form. Such drug therapy is effective only when the dopamine produced nerve terminals and AADC remain active and valid. If gene therapy is combined along with drug therapy even advanced Parkinson’s disease can be expected to produce a good result.
Single treatment will last a lifetime.
– How were the results?
We treated 6 patients in the trial started in 2007. The patients with treatment have recovered without exception.
The treatment is to have a 10 yen coin size hole in the head, then inject AAV vector from 4 needles in 2 holes. The amount placed in one hole is only 50microliters. PET (Positron fault imaging equipment) which can detect the enzyme made in the brain. Using this we examine the interior of the brain after treatment, and we determine that the enzyme is correctly made. The point I want to emphasize is, if treated once, the ability to synthesize dopamine last a lifetime. You don’t need to replenish the gene on a regular basis; also there were no adverse effects on the brain for this treatment. We only place 50 microliters AAV vector into four places.
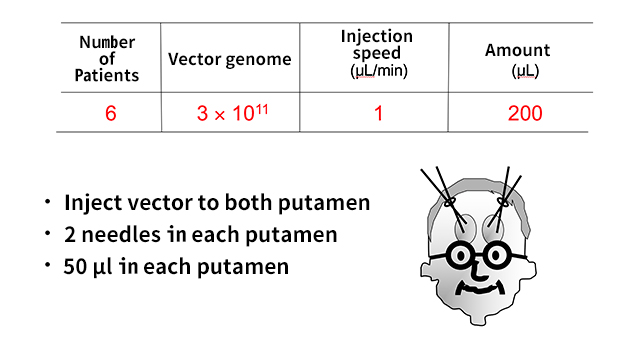
(Provided by professor Muramatsu)
Here is another example. There is a disease called congenital AADC deficiency, causing similar symptoms to Parkinson’s disease. Only a few hundred people have this disease in the world, but Taiwan has about 30 patients. This disease affects generations in Taiwan causing gene mutations.
In 2007, Taiwan’s doctors who watched the news reporting our result for the Parkinson’s disease treatment came to Jiti Medical University with patients (children). However we cannot easily treat them with gene therapy, because clinical trials of gene therapy can only be carried out with the approval of government. Therefore we provided them raw material to make the vector, and collected donations in Taiwan. We then commissioned United States venture companies to make the vector.
Treatment changed the life of the child.
Then the patient’s condition suddenly changed in 2010. I flew to Taipei and treated her. After treatment, the bed-ridden patient recovered, and now she can stand up, or even walk. Because of these good results, now we provide treatment to patients with congenital AADC deficiency in Japan. A patient in Kanagawa, originally her symptoms were light though now she can attend normal school. No medical institution in the world can treat like this, so we intend to treat a child patient coming from Australia in February. Further requests are coming from Russia and Canada.

Left: Patients and the family. Right: At the time of treatment.
– Her life was positively changed by treatment
I think that was a really good treatment.
And back to the Parkinson’s disease, we’ve been working out the treatment that added functions of AADC production. In order to make dopamine from food derived from tyrosine the body needs the ability to make TH and GCH. It is possible that the genes that produce these two features go into a single AAV vector. If it is successful, patients will live without medicine.
We are planning a clinical trial for this. The experiments using monkeys were already successful. The monkey treated 15 years ago just died the other day, we dissected it and confirmed that the gene worked for15 years.
Enzymes that dissolve the causative agent of Alzheimer’s disease.
– I can hardly wait! Are there more application examples for other diseases?
In experiments using animals, it is known that it is effective on various kinds of diseases.
For example, the cause of Alzheimer’s disease is believed to be due to the accumulation of a peptide called amyloid beta (Aβ) in the brain. Age spots called senile plaques are appearing on the brain. RIKEN Dr. Takaomi Saido made a discovery to begin the treatment for this. He found that the enzyme neprilysine dissolved senile plaques. If the enzyme works, you can treat it using AAV vector with the neplysine producing gene. In fact we did this experiment using rats ten years ago, and the rat brain spots have all disappeared.
However, for the therapeutic target range throughout the brain, it required making many holes in the brain, and you can’t do that realistically, so research was stopped. A few years ago a vector was developed to introduce through the blood vessel (Figure 4). In general it is difficult to spread the drug throughout the brain, however using this improved AAV, it goes well. This technique was patented. We have already confirmed the effect in mice and are thinking to apply for use with patients in the future.
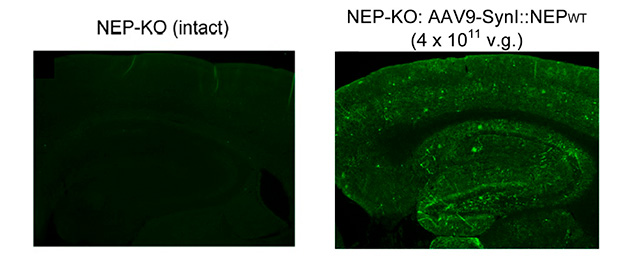
Left: Before the introduction. Right: After the introduction. Green indication is the part where AAV vector has gone through (reprinted from Science Report)
However for the practical application, a number of extremely high clinical trials have to be cleared. Because of the number of patients with Alzheimer’s disease, we need to have enough data from trials with a huge number of patients. Also to ensure effective recovery, we need to track for many years. These clinical trials take a huge time and cost big money. For this reason, even though it is extremely important in the aging society, unfortunately the clinical trials are on the back burner.
Establishment of treatment in incurable diseases is highly in demand. ALS; some hope has now come into light.
– Can you do something?
The disease that has a higher sense of urgency and briefly indicates the safety of gene therapy treatment is ALS motor neuron death disease.
The real cause of ALS is not well understood. However professor Shin Kaku at the Tokyo university medical research laboratory discovered a decrease of the enzyme called ADAR2 as a cause of motor neuron death. He revealed that the ADAR2 lacking mice lose motor neuron function the same as ALS.
Then we confirmed that motor neuron loss is reduced by my invented AAV vector with ADAR2 producing gene through blood vessels. This has opened the way to treat ALS. Placing AAV vector from the blood vessels is inefficient because they go around the body. So we experimented introducing from the cavity in the spinal cord which could be needled from the back of the monkey. We confirmed that the introduction from spinal cord to the brain is possible. We also confirmed using a pig that the length of the spinal cord is about the same as that in people. AAV vector to treat ALS is currently in preparation, we hope to start clinical trials in one year (Figure 5). At least we can start trials within the next year, I believe.
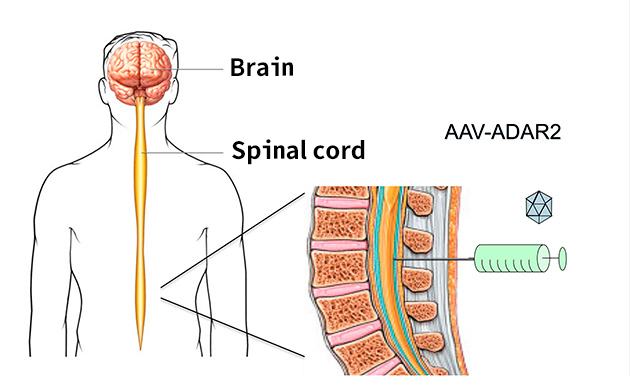
(Provided by professor Muramatsu)
Gene therapy can be applied to diseases other than neurological disease
– Gene therapy is available for most diseases as long as an enzyme involved. It is a highly versatile treatment.
That’s right. The available size of gene for AAV vector is 4 kb or less though, fortunately most of the genes for treatment fit this size. There are hundreds of child diseases due to enzyme deficiency, such as Mucopolysaccharidosis and Lysosomal Storage Disease. You can use it for haemophilia, retinitis pigmentosa,otolaryngology diseases, and also you can expect a positive effect for metabolic disorders of the liver.
There is also the idea to apply it as a preventative treatment, instead of using it after getting sick. For example anyone who is prone to Alzheimer’s disease with genes that could prevent the accumulation of the substance with prophylactic treatment, I guess.
– I’d like to prevent one now as well. Does it cost a lot for gene therapy treatment? Also, toward the practical use, what kind of problems do you have?
Honestly speaking, it takes a considerable cost to afford gene therapy at the moment. Because the production system is not quite ready yet, only Takara Bio Inc is making AAV vectors in Japan at the moment.
I believe that creating a system to quicken the flow from basic research to clinical trial is necessary. Prominent researchers who have been involved from the beginning of the AAV vector research in the West are now all affiliated with the enterprise company. In the west, it has been entirely shifted from the academic research phase to the industrialization phase. Attention has focused on how quickly the technology will be applied. Researching at the university is not catching up to the demands of the times. I feel that Japan is 10 years behind from the US in these movements.
To overcome this situation, I established the venture company Gene Therapy research co Ltd, collaborating with Toshihiko Sato a Radiology specialist and also a Director and practitioner at Utunomiya Central Clinic. We are based on recovery medical industrialization practice, the Life Innovation Centre in Kawasaki, Kanagawa Prefecture.
We established the venture company to progress early practical application
– What kind of activity is Gene therapy Institute doing?
We are working jointly with Takara Bio Inc, management on test drug manufacturing and creating a GMP vector which has cleared quality control standards. However that’s not good enough to catch up with the demands of the present age. Because of the wide range of applications, the demand far exceeds current production capacity. Therefore, we are making production facilities at our own expense. In future we’d like to organize a system to make vectors to meet the greater demand, along with Takara Bio Inc. We’d like to make at least 100 lines.
– First of all, what kind of disease treatment is targeted to produce the vector?
It is technologically advanced, but we are aiming to tackle the production of vector for ALS. The reason we say it is technologically difficult is because it may not work unless all targeted cells received the vector. Because of this, I think it is the most difficult disease for practical application. If you think as a business, it may be more reasonable to leave ALS on the back burner. However from a humanitarian standpoint as a doctor, I’d like to tackle ALS, patients are dying one after another while waiting for practical use. Fortunately Japan medical research (AMED) understands our point, and funded the research costs.
Also, you can make enough for 1000 people simply by moving the line only once if needed in a small amount to introduce in the part of the brain, as AAV vector for Parkinson’s disease. I’d like to work on this production. As production technology progresses steadily, production costs will decrease and will become more affordable, it will be less than 1 million yen to treat Parkinson’s disease in the future. Current surgery of Parkinson’s disease is to use electrodes to suppress symptoms, which costs more than 3 million yen, but certainly gene therapy will be more affordable than that. It’s not only cheaper but will last a lifetime. I think after 10 years, gene therapy will be available routinely.
The commercial application of gene therapy is in great demand by society.
– I think radical treatments such as gene therapy contribute to the solution of social problems especially the medical insurance system which is now in danger of collapse.
I agree with you. I think the situation of gene therapy will dramatically improve in 5 to 10 years. The time had come to change the business model of health-care related companies and organizations. Gene therapy will meet the needs of new era, I think.
In Japan many people pay attention to the use of iPS cells, enacted “The law on ensuring safely of regenerative medicine, etc“ so – called Regenerative therapy. The “etc” of regenerative medicine, etc refers to gene therapy. The preparations we make become practical and faster than ever within the framework of regenerative therapy. I believe the game has just begun.

Profile
Shinichi Muramatsu
Oriental Medicine Division, Jichi Medical University, regional medical center and Professor of Neurology internal medicine department. Tokyo University Medical Sience Institute of gene and cell therapy Centre Professor, Gene therapy Reseach Institute Board of Directors.
Born in Takasaki City, Gunma prefecture in 1983 and graduated from Jichi Medical University. After engaging the medical community in Gunma prefecture, research of the basal ganglia physiology. After Nagano Haramachi rural medical director, the scholar of the United States National Institute Health (NIH), and initiated a study of gene therapy. In 1997, after returning home, he developed gene therapy for Parkinson’s disease, amyotrophic lateralscleosis, Alzheimer’s disease, etc. He does advanced medical research such as gene therapy, artificial intelligence developed for the general medical system, whilst also using Chinese medicine in clinical practice.
Writer
Motoaki Ito
Enright CEO.
As an engineer of the Fujitsu semiconductor development for 3 years, Nikkei Micro devices, Nikkei Electronic, Nilkkei BP semiconductor research reporter and desk editor for 12 years. Experienced VA business activities as a techno associates consultant of joint think tank of Nikkei BP and Mitsubishi Corporation for 6 years, and marketing support activities as an advertising producer of Nikkei BP technology information groups advertising department for 4 years. In 2014, he established Enright Co, Ltd. Providing support service specializing in technology marketing, how to communicate more efficiently to specific companies about the value of technology.









